It is such a good method of organizing and presenting the known elements that it has been used to successfully predict the existence of certain elements. What does the atomic mass of an element depend on.

2 Saved Resources Writing Graphic Organizers Graphic Organizers Words
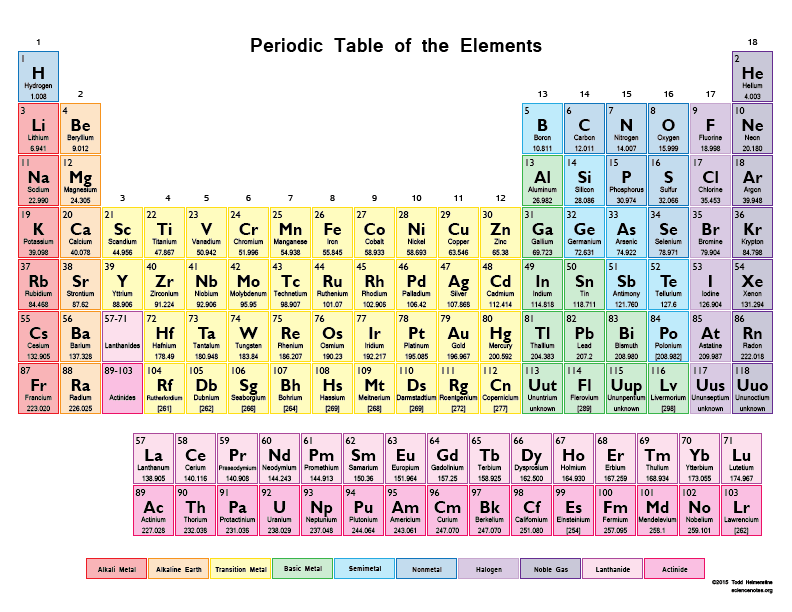
Rows called periods in order of increasing atomic number.

. In the modern periodic table the elements are arranged according to their atomic number not their relative atomic mass. In the periodic table the elements are arranged into. Vertical columns called groups where the elements have similar properties.
Rows called periods in order of increasing atomic number. Which statement best describes the oxidation numbers of the atoms found in magnesium chloride. The difference between a group and a period in the periodic table is that a group is each columnfamily with similar properties and a period is each row.
By atomic number C. The alkaline earth metals. It is organized by the increasing order of theatomic number.
Which best describes your ability to work with exponents. It became organized by atomic mass instead of atomic number. In this worksheet we will practice defining groups periods and blocks and linking the properties of elements to their positions in the periodic table.
Vertical columns called groups where the elements have similar properties. Which of the following statements best describes how Moseleys research affected the periodic table. The second column from the right side of the table.
Calculate the Mass of a Single Atom or Molecule What is Atomic Mass. The Periodic Tableis a table made of 7 periods rows and 18groups columns. What statement best describes how the periodic table is organized.
Magnesium has a 2- oxidation number and chlorine has a 2 oxidation number. Which position contains the element with the highest atomic number. Elements are listed in numerical order by atomic number.
It became organized by each elements name instead of its symbol. The arrangement starts from Doberneir law of triads then to Newlands law of octave then Mendleevs law where he arranged the elements based on their atomic weight. Calculate the Mass of a Single Atom or Molecule What is Atomic Mass.
The uppermost complete row of the table. Rows called periods in order of increasing atomic number. It made it necessary to determine these details before classifying.
In the modern periodic table the elements are arranged according to their atomic number not their relative atomic mass. The periodic table is a tabular array of the chemical elements organized by atomic number from the element with the lowest atomic number hydrogen to the element with the highest atomic number oganesson. By atomic mass B.
The atomic number is the number of protons in an atom of that element. In the modern periodic table the elements are arranged according to their atomic number not their relative atomic mass. The elements are arranged so that elements in a group all have the same valence subshell and thus have similar properties.
Where are the most reactive metals located on the periodic table. The column at the far left side of the table. The atomic number of an element is the number of protons in the nucleus of an atom of that element.
The periodic table provided the template for classification of elements by their chemical properties and atomic weight. Mendeleev created his chart between the years of 1868 and 1870 as he was writing his book titled The Principles of Chemistry. Magnesium has a 2- oxidation number and chlorine has a 1 oxidation number.
Which best describes a substance. All elements in a period can have up to the number of electrons as there are. How is the periodic table organized.
Which best describes how the current periodic table is arranged. In the periodic table the elements are arranged into. Vertical columns called groups where the elements have similar properties.
Be sure to give examples as well as the definition. In alphabetical order by element symbol D. Atomic mass is a value that depends on the distribution of an elements isotopes in nature and the masses of those isotopes.
It lists them in order of patterns of atomic weight electron configuration reactivity and electronegativity. According to which property are the elements in the modern periodic table organized left to. All elements in a period have the same number of electrons as there are elements in the period.
The Periodic Table Ways the Periodic Table is Organized Use the Chemical Interactions textbook to describe the following ways the periodic table is organized. It became organized by atomic number instead of atomic mass. Heres how it works.
Most of the mass is contained in the nucleus of the atom The periodic table is useful because it is organized to show many patterns and trends. The lanthanides and actinides. The periodic table is an arrangement of elements based on particular properties.
Which best describes your participation in discussions about math problems or topics. The organization of the periodic table allows you to predict the properties of the elements based on their position on the chart. The middle column on the periodic table.
Magnesium has a 2 oxidation number and chlorine has a 1- oxidation number. Which statement best explains how periods on the periodic table are organized. In the modern periodic table the elements are arranged according to their atomic number not their relative atomic mass.
So element number 1 hydrogen is the first element. The elements are organized by atomic number increasing periods rows and groups columns. The uppermost complete row of the table.
Rows called periods in order of increasing atomic number. The periodic table is organized by periods and groups. The rows at the bottom of the table connecting two section.
Vertical columns called groups where the elements have similar properties. The modern periodic table organizes the known elements in several ways. Group 2 and Period 3 Which statement best describes the mass of an atom.
The Periodic Table Chemistry 7th Grade.
Is There A More Elegant Way To Arrange The Elements Of The Periodic Table Quora

0 Comments